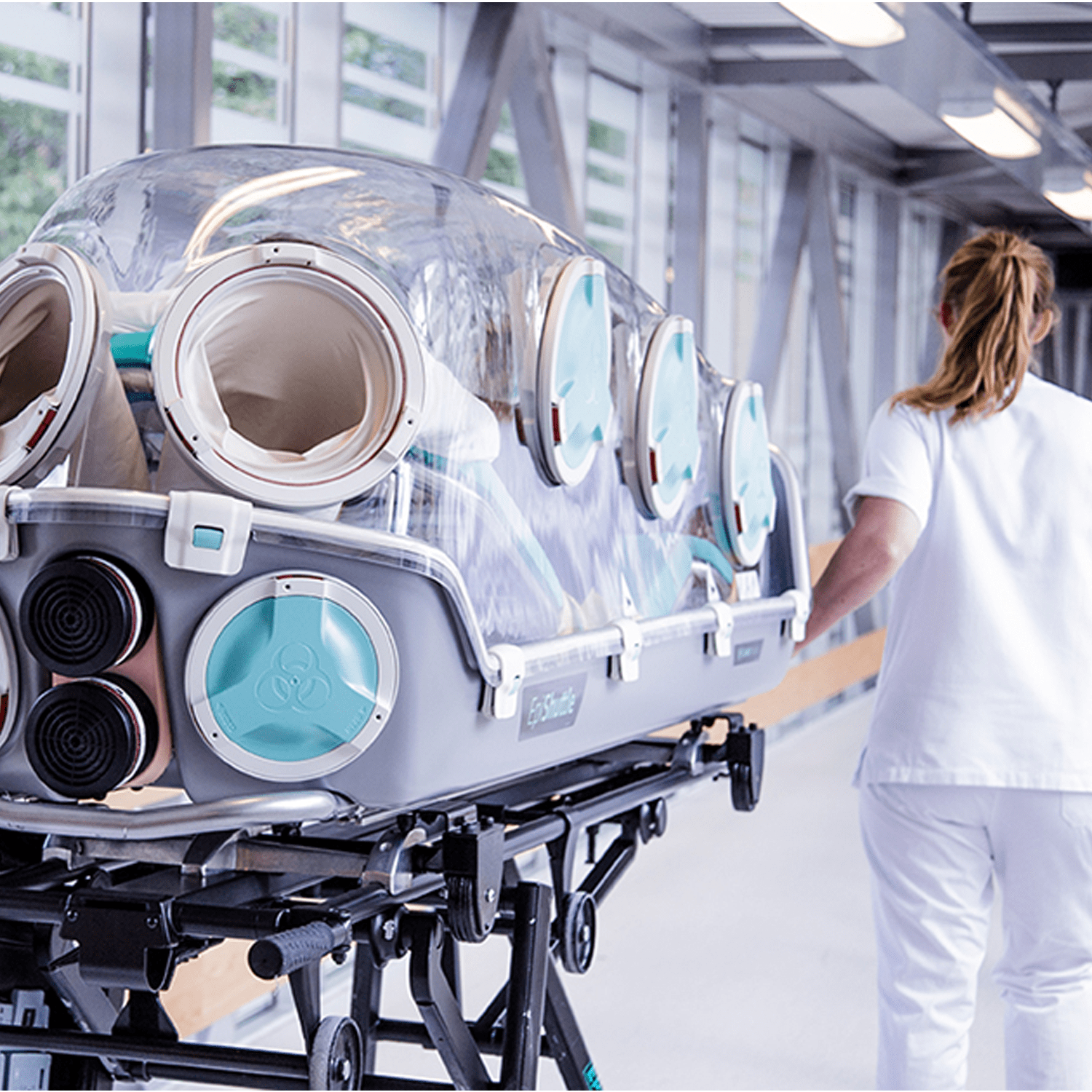
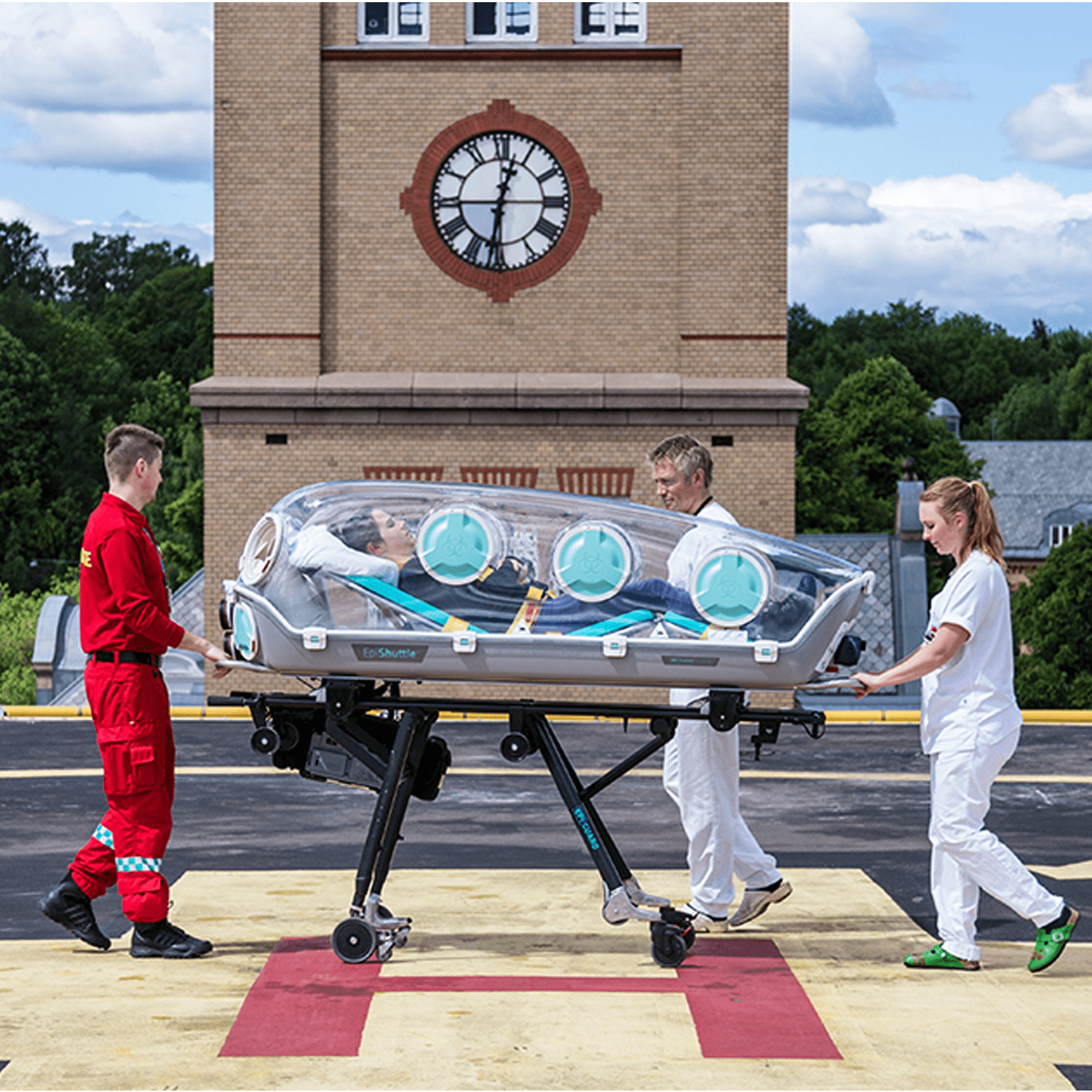
A unique medical isolation transportation system
EpiShuttle is a medical isolation and transportation system designed for optimal safety and numerous treatment options during patient loading and transport. The unit is a single-patient isolator made of solvent-resistant materials. EpiShuttle can be carried and is compatible with leading ambulance-stretcher undercarriage EMS systems.
The EpiShuttle is compatible with most mechanical ventilator circuits. Using negative pressure and P3 filter, the isolator provides full environmental protection from particulate cross-contamination of highly infectious diseases. In positive pressure mode, the isolator protects patients from hazardous environmental agents through the use of CBRN filters. The design allows for full intensive care treatment and emergency procedures, such as intubation and insertion of central venous catheters. The EpiShuttle is designed for multiple use, easy communication and optimal patient comfort.
The EpiShuttle has been created by clinicians with many years of experience within the field. Every little detail and function of the device are thoroughly designed and thought through and tested, from patient handling to facilitating advanced medical procedures. This has resulted in a device uniquely designed to meet the needs of the patient, yet specifically the healthcare providers ability to give the absolute best patient care without the risk of contamination. In fact, the EpiShuttle has been designed by users for users.
EMS system compatible
EpiShuttle has an L-Track attachment system that allows for quick and secure attachment to stretchers, ambulances, aircrafts, and helicopters.
ON – ite treatment
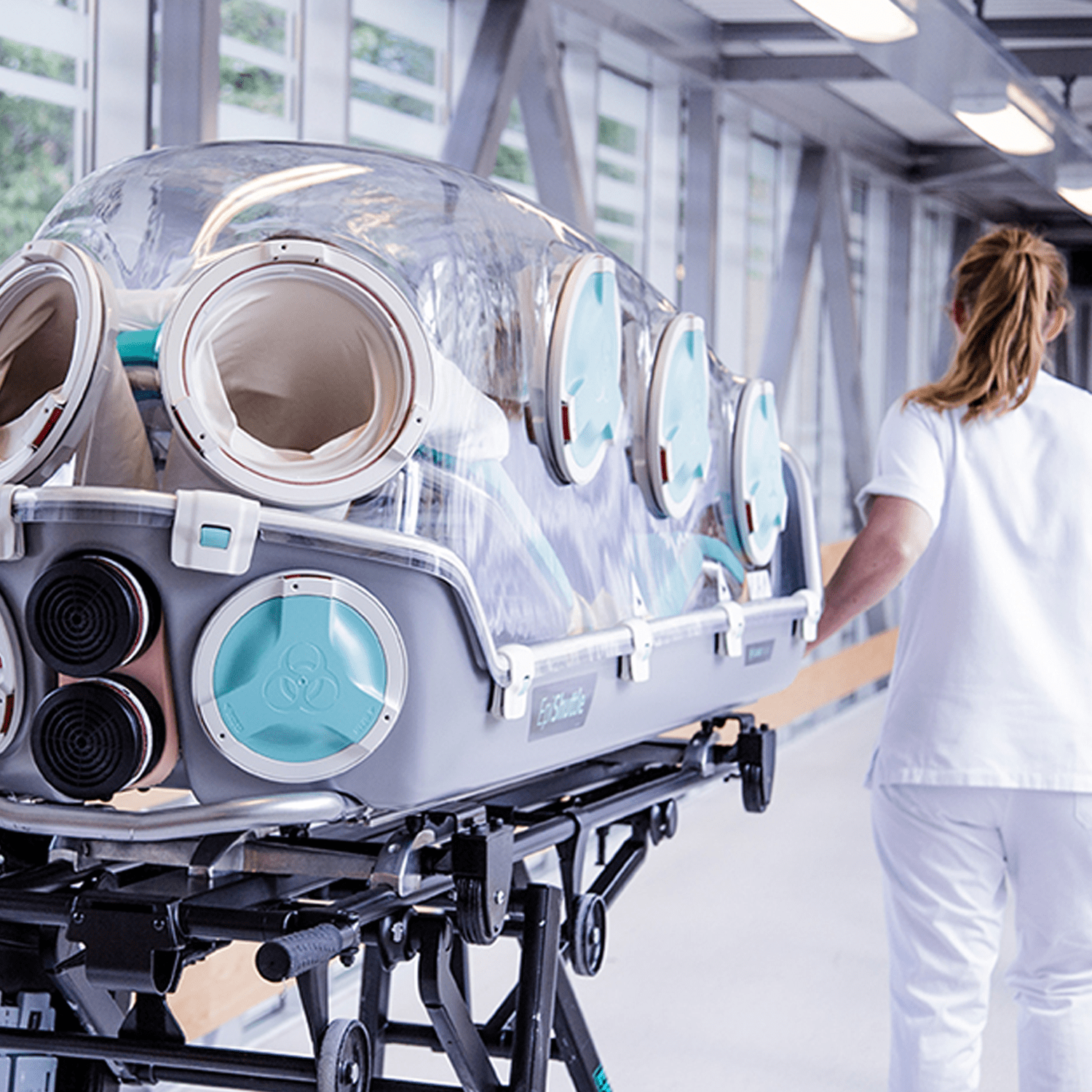
For optimal treatment during transport, the EpiShuttle has eight entry ports. These ports have sealed gloves or other options like sluice port and waste bag that can be changed during use.
Reusable
The device is simple to clean and disinfect for multiple use. The device is easily disassembled and assembled without the use of any tools.
Quick patient loading
Short patient loading time possible due to easy operations and intuitive functions. The hard top is easily secured with EpiShuttle´s unique locking system to obtain airtight connection.
Battery powered
EpiShuttle allows for uninterrupted power for a minimum of 6 hours. The batteries can be replaced during transport, keeping the patient and environment safe from contamination up to 24 hours.
Dual protection system
The pressure modes can be set to either protect the environment from the infected patient, or protect the patient from contaminants in the environmental air. It complies with all levels of transmission based precautions; standard, contact, droplet and/or airborne.
Intensive care
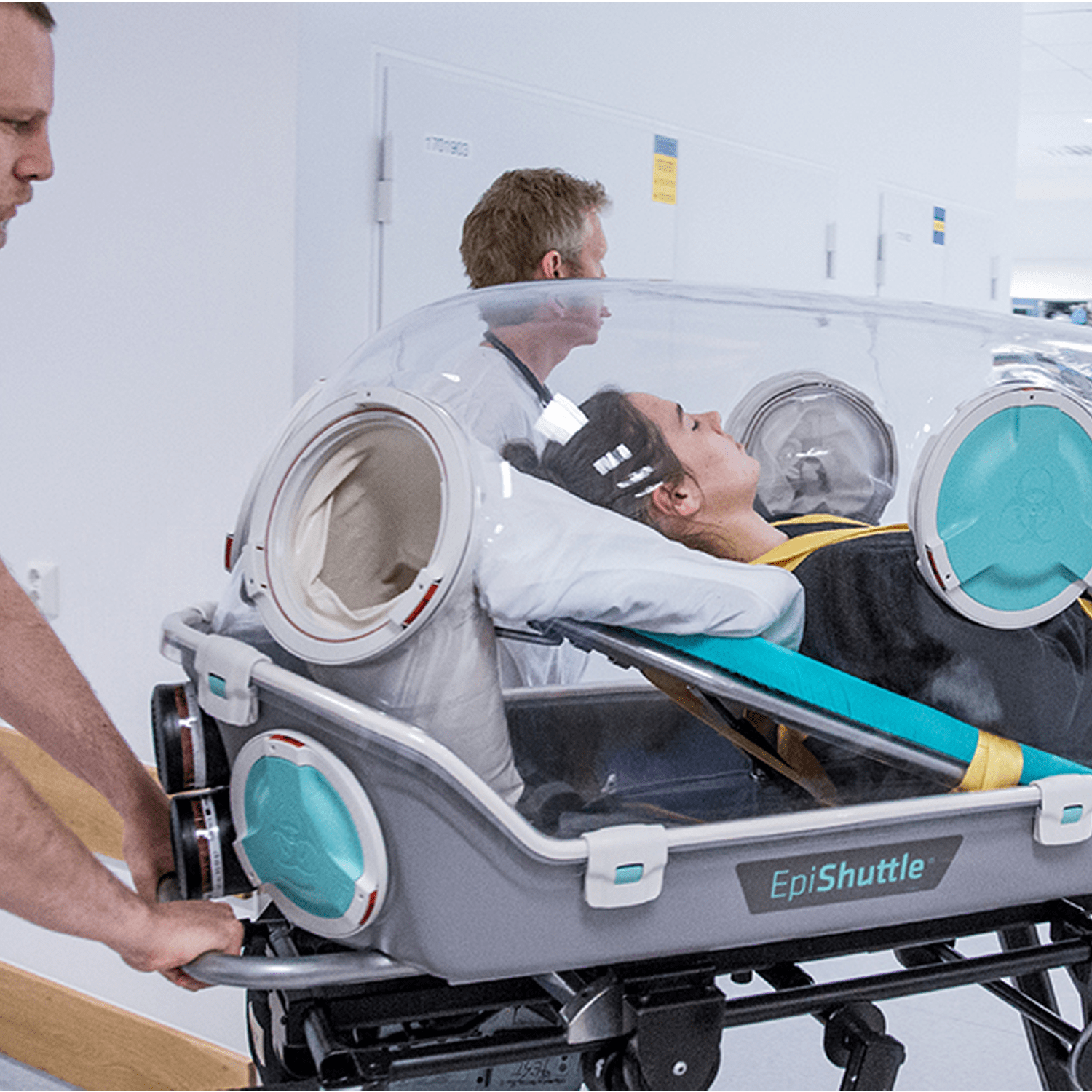
EpiShuttle is equipped with medical ports for connecting IV lines, oxygen lines and monitoring cables, as well as providing mechanical ventilation to the patient. The intensive care system allows the health personnel to monitor, medicate, and give treatment to the patient without direct exposure.
Optimal patient comfort
EpiShuttle has been uniquely designed for optimal patient comfort. The integrated bed is adjustable both for knee angle and backrest, allowing for increased comfort for the patient and better conditions for clinical procedures. The visibility through the transparent hardtop allows for better communication between the patient and surroundings.
EpiGuard has tested materials used in the EpiShuttle with several disinfection chemicals.
Disinfectant that are EpiShuttle Compatible:
Disinfectant that is NOT EpiShuttle Compatible is:
EpiGuard have developed and validated two procedures for disinfecting the EpiShuttle with either peracetic acid or hydrogen peroxide vapor. The chemical compatibility matrix may act as a guideline for customers who want to implement their own disinfection procedures with their local hygiene department
Caution: ensure proper removal of chemical residues after decontamination, as these residues deteriorate the materials.
Service is essential to keep medical equipment operational and safe. Our Nordic service team consists of experienced service technicians stationed across the Nordic countries. Our service technicians are ready to take on your every service need.”

Nasal PAP ventilation system
The SuperNO2VA nasal PAP ventilation system uses the flow from any oxygen source. Delivers positive airway pressure to stent open the upper airway, allowing for the preoperative delivery of positive pressure ventilation and oxygen for patients with a decreased level of consciousness. The SuperNO2VA™ nasal PAP ventilation device is available in medium and large sizes and is offered as a standalone mask with a head strap and as a system, kitted with a hyperinflation bag. These configurations offer flexibility as it allows the SuperNO2VA™ nasal PAP ventilation device to be used with either an anesthesia machine or with only an oxygen flow meter.

The compact and intuitive AG CUFFILL device is the most accurate solution for measuring both pressure and volume of tracheal cuffs in all conditions. Its pocket-size, syringe-like design allows a simple and easy operation by medical professionals, including first-responders and hospital staff, and by users in the home care environment.
Key Features

BiliSwaddler®, phototherapy with fiberoptic lightpads
BiliSwaddler alows you to practise development-promoting care practically anywhere, whenever it is in the newborn intensive care unit, nursery or at home. During the therapy session, the baby can be breast fed or held without it impairing on the bonding between the infant and parents. One Swaddler size for all commonly used phototherapy fiber optic pads such as GE Healthcare, Natus Medical, Atom Medical etc.
Soft cushioning (3D knit fabric) for pressure relief and support of moisture management for optimum climatic control. BiliSwaddler can be used in a baby bed or in the arms of a caregiver (kangaroo care). Innovative Interlock-fabric (80% CO, 20% PES Trevira bioactive®) with a permananet antimicrobial effect is gentle and prevents skin irritation. Please use appropriate eye-protection during photo therapy sessions.

Top-of-the-range activity monitor
The ActiGraph GT9X Link captures and records high resolution human activity information using a solid state 3-axis MEMS accelerometer and our proprietary filtering algorithm. The GT9X Link is our most sophisticated activity monitor and represents a significant upgrade from previous models. Building on our validated physical activity and sleep measurement platform, it has been redesigned with a rich set of new features along with multiple new channels of sensor data captured by gyroscope, magnetometer and secondary accelerometer. High resolution LCD screen, Bluetooth® for wireless data capture and uploads to ActiGraph’s mobile applications makes GTX9 Link the most versatile and user-friendly activity monitor in our range. It is fully backward compatible with previous device models to ensure data comparability for our existing clients.