Innovative phototherapy cover made of a high-tex material
BiliCap® is made of a 3-layered functional specialty knit fabric. A highly elastic, breathable membrane in the core assures the phototherapy cover’s imperviousness to light. The 3D knit spacing fabric underneath ensures optimum noise attenuation, provides an anti-slip effect and permanently retains the cover’s shape.
Additionally, the material is characterised by low weight and contains an active substance with permanently antimicrobial effect.
BiliCap® optimally shields the surrounding treatment places against the high light intensity while providing fast and flexible access via 4 side and 2 head/foot flaps. The one-size design allows flexible use, fitting all incubator models commonly available on the market. BiliCap® is certified according to Oeko-Tex® Standard 100, washable up to 95 °C and suitable for tumble-drying. Guaranteed permanence of function and colour even in the case of frequent laundering. Available in 2 colours with individual ornaments and embroidery.
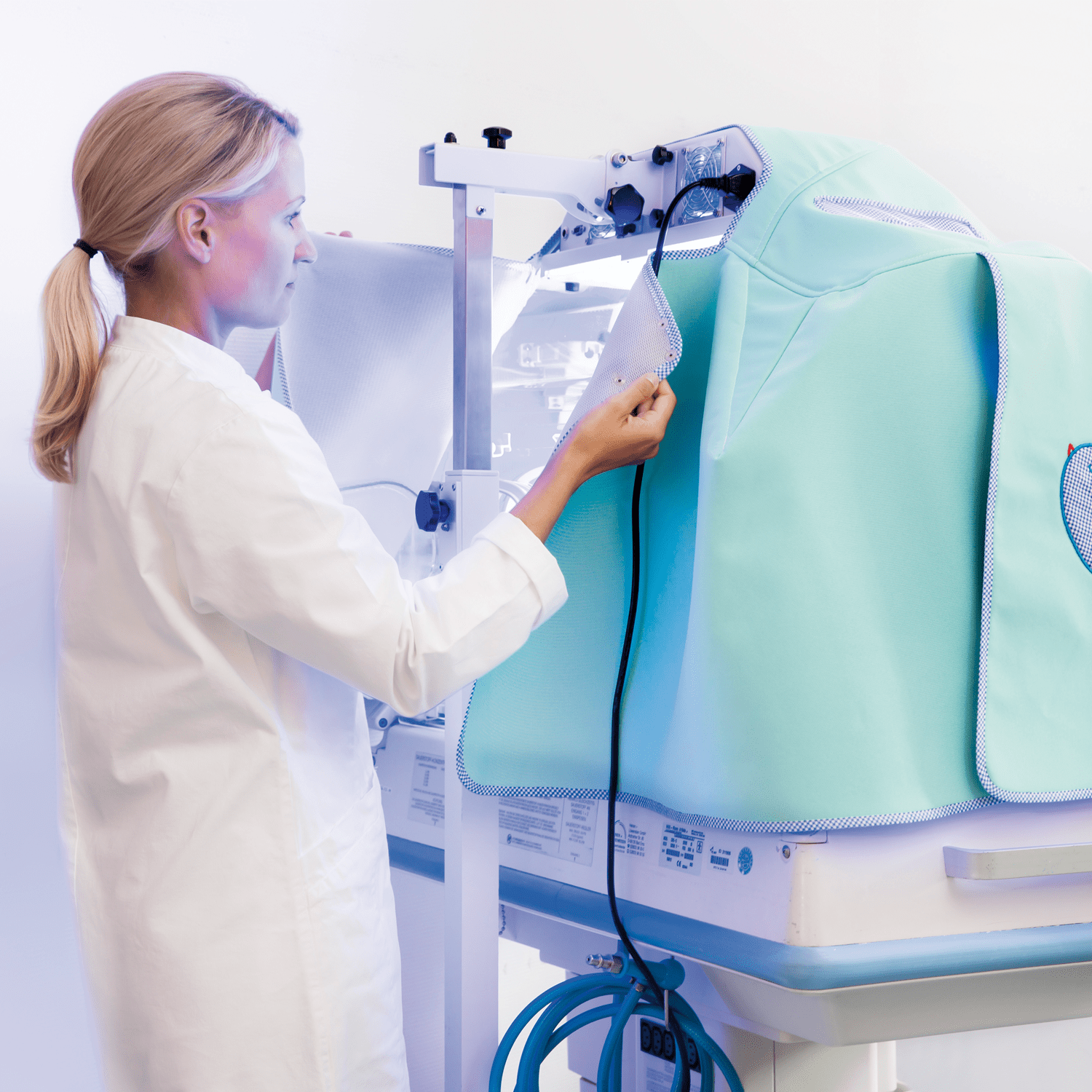
Numerous of properties have positive effects on the development of preterm babies. The complete light reduction (opacity) and outstanding noise attenuation provided by our incubator covers, for example, plus the long life of our products despite the stringent hygienic requirements in NICUs.
In the case of the positioning aids, 3D knit fabrics ensure optimal transport of moisture, a pleasant climate and perfect pressure relief. The very soft, fine-threaded microfibre material has a temperature-compensating effect and makes a feeling of pleasant, dry comfort possible at all times. Raw and auxiliary materials are selected from a pool of suppliers specialising in the field of medical products/engineering. All products are either manufactured in-house or by certified partners and can be customised to precisely suit your specific requirements.
Service is essential to keep medical equipment operational and safe. Our Nordic service team consists of experienced service technicians stationed across the Nordic countries. Our service technicians are ready to take on your every service need.”

Delivering Confidence and Simplicity during Neuromuscular Blockade
TetraGraph is a quantitative neuromuscular transmission monitor based on electromyography (EMG). The TetraGraph monitor stimulates, measures, analyzes and displays muscle function in surgical patients receiving neuromuscular blocking agents (NMBAs). NMBAs are used in almost 50% of surgical cases. Inadequate reversal of NMBAs can lead to residual neuromuscular blockade (RNMB). RNMB delays recovery and can lead to life-threatening complications. To prevent complications, quantitative neuromuscular monitoring is rapidly becoming the standard of care when NMBAs are administered.

The dual-valved LiteAire MDI holding chamber: Collapsible, disposable paperboard design.
The LiteAire® collapsible MDI holding chamber is a unique MDI holding chamber and an innovative alternative. LiteAire’s unique dual-valved MDI holding chamber design delivers pop-up convenience and effective drug output at a fraction of the cost of most plastic holding chambers. In many clinical settings, the LiteAire can reduce costs by replacing existing rigid plastic holding chambers or inefficient spacers with a paperboard alternative. The unique design allows the LiteAire to be reused by a patient over multiple doses and meets and often exceeds the performance of plastic holding chambers.

Insight Watch – Medical-Grade actigraphy monitoring, redefined
The compact and stylish CentrePoint Insight Watch captures and records continuous high-resolution raw acceleration data, sampled up to 256 Hz, to support the development of novel endpoints related to real-world activity, mobility, and sleep behaviors to provide objective, real-world physical activity, mobility, and sleep measures, in near real time.
Used in conjunction with the CentrePoint Data Hub or mobile application, captured data is passively transmitted to the cloud for near real-time detection of compliance issues and potential data trends.

Groundbreaking aerosol drug delivery
Aerogen Solo delivers more effective and controlled treatment, lowering risk of exposure. The Aerogen Solo delivers more inhaled medication to the lungs⁴, in less time ⁵, with less residual volume,7 compared to jet nebulizers, giving confidence in a more effective and controlled treatment.
The medication reservoir is isolated from the breathing circuit which minimizes nebulization of contaminated fluids¹. The risk of exposure to bioaerosols is reduced because the need to open the circuit is eliminated⁸.
The Aerogen Solo delivers more effective medication throughout the hospital through mechanical ventilation and non-invasive support such as non-invasive ventilation (NIV), high flow therapies (HFNC) and spontaneously breathing patients¹⁻⁷.
The Aerogen Solo is uniquely designed remaining in place during ventilation⁸. This closed system requires minimal management and can be left inline reducing staff handling and workload⁷ ¹⁰. Easy to set up and virtually silent operation, with no added flow required⁷.